Abstract
Introduction: Extramedullary disease (EMD) in multiple myeloma is recognized to be a feature of aggressive or advanced disease and known to have a poorer outcome than myeloma with paramedullary or intramedullary disease. However, due to variability in its definition and lack of consistency in reporting in published literature, prognosis and treatment outcomes are not well characterized. In this study, we reviewed EMD at presentation for CAR-T cell therapy and emergence or relapse of EMD post CAR-T administration. For this review, we have defined EMD as soft tissue plasmacytoma(s) arising outside of bone marrow (BM) and excluded intramedullary and paramedullary soft tissue masses contiguous to bone.
Methods: We conducted a retrospective review of 85 patients with relapsed refractory multiple myeloma who underwent a CAR-T procedure either on a clinical trial or with a commercially approved product at our institution from April 2017 - March 2022 with a minimum of a 3 month follow up. Kaplan Meier and reverse Kaplan Meier methods were used to compute time-to-event and median follow up calculations. This study has been approved by the Mount Sinai IRB.
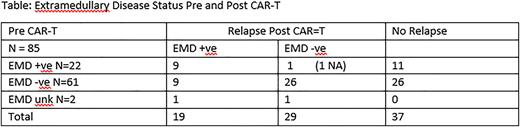
Results: EMD was present prior to CAR-T in 22/85 (25.9%) patients and 61/85 (71.8%) had BM involvement. At a median follow up of 35 months (range 1-39 months), 48 (56.5%) patients have relapsed, including 19/85 (22.4%) with EMD. Median PFS for the overall group was 18 months, and for the patients with EMD +ve disease prior to CAR-T was 12 months.
Of the 22 patients with EMD pre CAR-T, 11 (50%) have relapsed; 9 have relapsed with EMD (40.9%), 1 has relapsed without EMD, and 1 had no PET info at relapse. 6 of the 9 EMD relapses occurred at the previous sites of disease, three patients, however, had isolated areas of relapse which were new compared to original areas of EMD involvement, including malignant pericardial effusion in one and leptomeningeal disease in another.
12/22 patients (54.5%) who were EMD +ve pre CAR-T are EMD -ve post CAR-T (see table) 5 of the 11 non-relapsed patients remain in EMD -ve complete remission (CR) at 22+ months of follow up.
19/48 (39.6%) patients had extra-medullary disease (EMD) at relapse post CAR-T. Only 9 of these (47.3%) had EMD pre CAR-T; 9 were -ve for EMD pre CAR-T and 1 patient had no PET info prior to CAR-T. (see table)
Of interest, 7 of 19 patients who had EMD +ve at relapse were BM MRD -ve (36.8%), and 2 additional patients were BM -ve, MRD test NA.
35 patients relapsed in the bone marrow post CAR-T. The majority of these had BM involvement pre CAR-T (31/35, 88.6%), unlike in EMD relapses in whom almost half did not have EMD pre CAR T (9/19, 47.4%).
Conclusions: In this retrospective study looking at patterns of disease relapse for EMD vs BM in CAR T patients, we found that 50% of advanced patients with EMD prior to CAR T were in remission post CAR T, with 5/11 non-relapsed patients remaining EMD -ve at 22+ months. Over half of EMD +ve (54.5%) prior to CAR-T, were EMD -ve post CAR-T, showing that CAR-T has the potential to benefit even advanced patients who have EMD which is a known risk factor for shorter duration of response and progression free survival. In our review, our patients with EMD prior to CAR-T did have a shorter median PFS than the overall group. While 3 patients had new sites of EMD relapse, the majority relapsed at sites of original disease. In the former cases, this raises the question of whether there are immunologic sanctuary sites that are susceptible to tumor relapse. On the other hand, more aggressive local control such as the addition of radiation therapy to sites of bulky disease may help to prevent relapses in sites of previous EMD. At our institution, we are performing spatial transcriptomics to relate disease features and the micro environment of EMD lesions to better understand this.
Additionally, although the BM cleared quickly by month 1 post CART with negative MRD testing by next generation flow or NGS with sensitivity of at least 10-5, the majority relapsed in the BM. BM MRD testing did not pick up 36.8% of proven EMD positive relapses.
As there is paucity of data for treatment specific outcomes for EMD, well designed prospective trials would be important to study this systematically, in conjunction with translational studies, to provide evidence based treatment guidelines.
Disclosures
Richard:C4 Therapeutics: Research Funding; Karyopharm: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding. Lancman:Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Rossi:gsk: Consultancy; adaptive: Consultancy; sanofi: Consultancy; BMS: Consultancy; janssen: Consultancy. Chari:Pharmacyclics: Research Funding; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy; Oncoceutics: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Array Biopharma: Research Funding; Novartis Pharmaceuticals: Research Funding; Glaxo Smith Klein: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees. Sanchez:Takeda: Consultancy. Rodriguez:Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Cho:Takeda: Other: Receive laboratory research support from the above companies. Salary value is less than $10,000 per company., Research Funding; BMS/Celgene: Other: Receive laboratory research support from the above companies. Salary value is less than $10,000 per company., Research Funding. Richter:BMS/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Secura Bio: Consultancy, Honoraria; Takeda: Consultancy; Oncopeptides: Consultancy, Honoraria. Verina:BMS: Speakers Bureau; karyopharm: Speakers Bureau; janssen: Speakers Bureau; sanofi: Speakers Bureau. Escalon:Rigel Pharmaceuticals: Speakers Bureau. Jagannath:Karyopharm: Consultancy; BMS: Consultancy; Sanofi: Consultancy; Janssen Pharmaceuticals: Consultancy; Takeda: Consultancy; Legend Biotech: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal